Ocean Acidification: A chemical reaction
Ocean Acidification: A chemical reaction
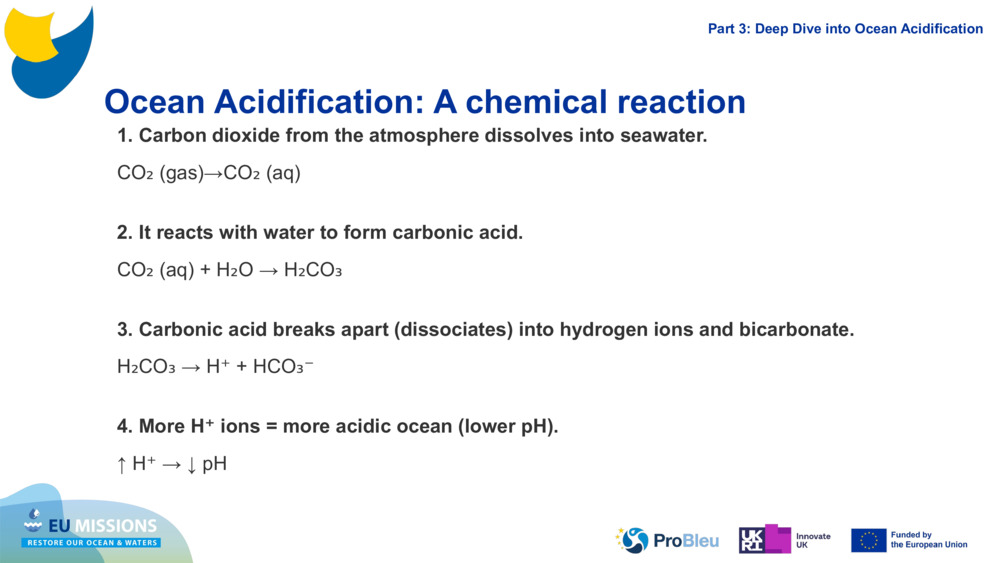
Ocean Acidification: A chemical reaction 1. Carbon dioxide from the atmosphere dissolves into seawater. CO₂ (gas)→CO₂ ( aq ) 2. It reacts with water to form carbonic acid. CO₂ ( aq ) + H₂O → H₂CO₃ 3. Carbonic acid breaks apart (dissociates) into hydrogen ions and bicarbonate. H₂CO₃ → H⁺ + HCO₃⁻ 4. More H⁺ ions = more acidic ocean (lower pH). ↑ H⁺ → ↓ pH Part 3: Deep Dive into Ocean Acidification
Original Slide Deck: Water Chemistry with Plymouth Marine Laboratory
Topics: Sustainable Resources. Deoxygenation. Ocean. Freshwater. Ocean Chemistry. Nutrient Pollution.
Suitable Ages: 12. 13. 14. 15. 16. 17. 18.
Keywords: Acidity. Alkalinity. Biology. Carbon. Carbon Dioxide. Chemical Reactions. Chemistry. Eutrophication. Farming. Hypoxia. Indicators. Molecules. Nutrients. Ocean Acidification. Ph.
Uploaded By: pml-admin
Number of bundles using this content: 1
Licensed under CC BY 4.0
This content has been used in the following resource bundles:
Water Chemistry with Plymouth Marine Laboratory
Teaching about water chemistry is important because it connects key sc...
View Science Story